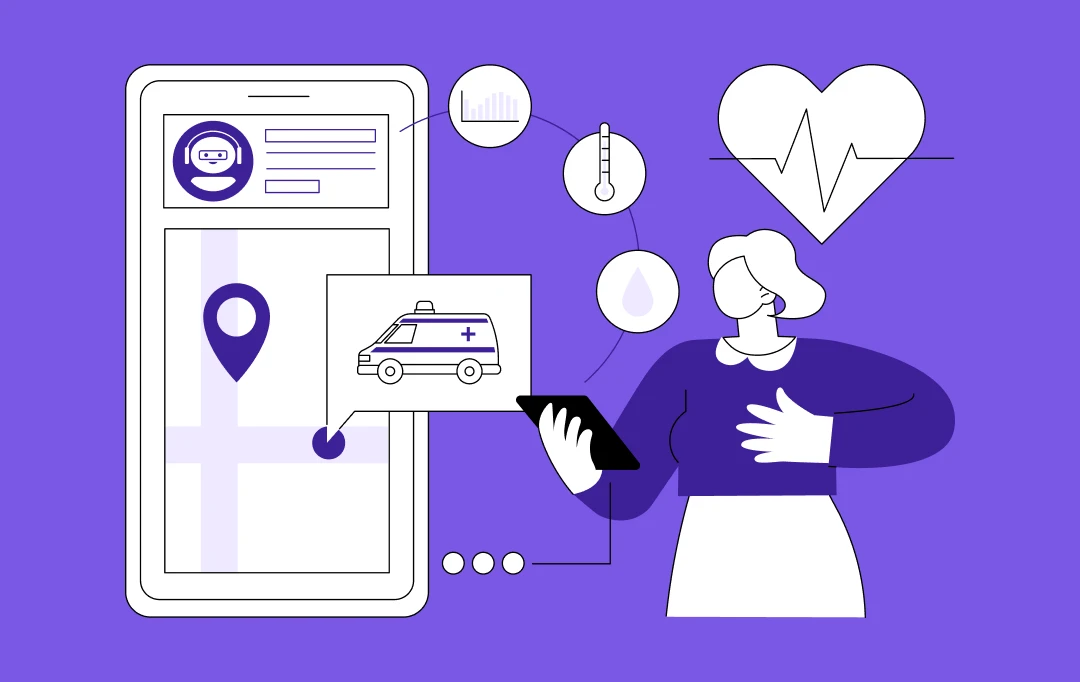
NEW YORK, – July 16, 2025 – Appinventiv, the world’s leading healthcare app and software development service provider with over 10 years of industry experience, is proud to announce its recent partnership with five behavioral health providers to develop telehealth platforms that meet the requirements of 42 CFR Part 2, as mandated by SAMHSA.
With unmatched experience in providing development services to over 200 healthcare companies, the partnership and the developed solution offer the highest level of security, scalability, and patient access, empowering trust and compliance for substance use disorder (SUD) treatment.
Expertise in SAMHSA Compliance
The core challenge of 42 CFR Part 2 is its uncompromising stance on data confidentiality, which surpasses even HIPAA’s requirements. Appinventiv’s expertise translates directly into the solution’s architecture, which is engineered to enforce re-disclosure prohibitions and provide dynamic, patient-controlled consent. This focus on preventive compliance ensures our partners can deliver critical SUD care while being protected from the significant legal and financial risks associated with a data breach.
Data Security That Is Not Compromised:
- Uses AES-256 encryption for data-at-rest and TLS/SSL 1.3 for data-in-transit, providing the uncompromised protection of sensitive SUD patient records through partnerships with SAMHSA-accredited behavioral health champions.
- Uses multi-factor authentication (MFA) with biometrics and tokens to reduce the risk of unauthorized access by 99.9% according to industry standards, making compliance with 42 CFR Part 2 worry-free.
Effortless SAMHSA OTP Compliance:
- Empowers providers with SAMHSA’s opioid treatment program (OTP) flexibilities, including initiating buprenorphine or methadone via audio-only or audio-visual telehealth.
- As initial in-person examination barriers are minimized, comprehensive evaluations with a 14-day physical exam compliance are conducted.
- Automates compliance workflows to ensure SAMHSA’s rigid standards are met so that providers can focus solely on care delivery, not regulatory workloads.
Scalable, Future-Ready Architecture:
- Utilizing AWS and Azure cloud service infrastructure, the developed solution can scale from 10 to 10,000+ concurrent users, running at 99.99% uptime, and has been successfully stress-tested with clients.
- The solution features a modular, Kubernetes-based architecture, informed by over 10 years of knowledge gained from feedback from healthcare clients, resulting in updates that can take as long as 48 hours to ensure alignment with the ever-changing regulatory guidelines of SAMHSA and DEA.
State of the Art Security Innovation:
- Utilizing rigorous penetration testing tactics led by our CISSP-certified cybersecurity teams, 98% of potential vulnerabilities neutralize through simulated attacks, ensuring there are no operational gaps in security.
- Using zero-trust architecture enables our AI-driven threat detection to analyze and process over 10,000 data points per second, intervening with uncertain threats in milliseconds and achieving 25% fewer false negatives than traditional systems.
- We also use machine learning algorithms with 50 TB of healthcare data to offer and define proactive security that can anticipate and mitigate risk with predictability.
“We affirm an unwavering commitment to data integrity and patient privacy. Our SAMHSA-compliant solutions are built on a technically robust framework, leveraging granular access controls, advanced encryption, and secure architectures to rigorously protect sensitive behavioral health and substance use data. This deep technical foundation not only ensures stringent regulatory adherence but also cultivates profound user trust, enabling us to ethically expand our vital health services and mitigate critical business risks,” said Appinventiv’s VP of Security and Compliance.
About Appinventiv
Appinventiv, an expert in healthcare software development, has helped a worldwide portfolio of clients with over 3,000 developed, high-quality projects since its founding, each compliant with HIPAA, HITECH, GDPR, and HL7 regulations. As a respected force in the industry, our team of over 1,600 agile experts employs technology and leverages trends in AI, IoT, and blockchain to help clients develop innovative HealthTech solutions. These innovations, such as digital platforms for telemedicine, comprehensive EHR systems, and patient care mobility tools, are produced with the utmost attention to detail and precision to deliver transformational change in patient care and internal operations.


Cairo, Egypt – September 19, 2025 – Appinventiv, a global leader in AI-driven digital transformation and product development, today announced its strategic readiness to support Egypt’s National AI Strategy, which was recently unveiled in its second edition for the period of 2025–2030. The new strategy, with its vision of "Inclusive AI to foster Digital Egypt,"…

Appinventiv has been recognized as one of the Top Metaverse Development Service Firms in the list published by TrustFirms. The recognition has been awarded based on the organization’s tremendous efforts that have significantly impacted the metaverse ecosystem. Appinventiv has been revolutionizing the Metaverse development industry in the United Arab Emirates with its innovative ideas, cutting-edge products,…

The proliferation in the number of smartphones and tablets have led to evoking a keen interest in mobile apps by the companies. As the enterprises are continuously seeking for some means for boosting employee productivity, mobile apps opens up an avenue for spending your time and money, in a more fruitful way. Not only that,…