- Why Are Enterprises Investing in Medical Device Software Development Now?
- What Types of Medical Device Software Should Enterprises Build?
- What Is the Difference Between Software in Medical Devices and Software as a Medical Device (SaMD)?
- How Does the Medical Device Software Development Process Work?
- Step 1: Research and Discovery
- Step 2: Requirements and Risk Classification
- Step 3: Medical Device Software Design
- Step 4: Development and Implementation
- Step 5: Verification and Validation
- Step 6: Release and Controlled Deployment
- Step 7: Post-Market Monitoring and Lifecycle Support
- Which Medical Device Software Standards and Regulations Must Enterprises Follow?
- Why Risk Class Changes Everything?
- How Much Does Medical Device Software Development Cost for Enterprises?
- What Drives Medical Device Software Development Costs?
- What Are the Biggest Challenges in Software Development for Medical Devices?
- Regulatory Execution
- Legacy Integration
- Interoperability
- Cybersecurity and Data Privacy
- Organizational Readiness
- Should Enterprises Build, Buy, or Partner for Medical Device Software Development?
- Decision Criteria for Large Enterprises
- How to Choose the Right Medical Device Software Development Partner?
- What Future Trends Are Shaping Medical Device Software Development?
- Why Is Appinventiv the Right Partner for Medical Device Software Development?
- Frequently Asked Questions
Key Takeaways
- Software now defines medical device competitiveness, compliance readiness, and long-term enterprise growth.
- Regulated development lifecycles and standards alignment reduce approval risks and costly delays.
- SaMD and connected device software are unlocking new data-driven revenue models in healthcare.
- Cost, risk classification, and validation depth shape enterprise investment and delivery timelines.
- Build, buy, or partner decisions directly impact compliance control and time-to-market outcomes.
- The right development partner accelerates innovation while maintaining audit readiness and regulatory confidence.
Most enterprise healthcare leaders reach a moment where hardware innovation is no longer the main challenge. The real pressure sits elsewhere. The software running inside and around medical devices now determines how fast products reach market, how safely they operate, and how confidently regulators approve them.
In fact, 86% of healthcare organizations already report extensive use of AI, raising expectations for intelligent, connected medical systems. If your team is planning a new connected device or modernizing an existing portfolio, this shift is already on your roadmap.
Medical device software development today is not just an engineering task. It is a business-critical capability. One regulatory delay can stall a launch. One integration gap can disrupt hospital workflows. One security weakness can put patient trust at risk. You feel this reality when product, compliance, and technology teams sit in the same meeting trying to align timelines.
Software development for medical devices also demands decisions that go beyond code. What type of software should you build? How will you manage compliance? What will it cost? Which development model reduces risk without slowing innovation?
This guide walks you through the process, types, costs, and compliance expectations involved in building software for medical devices. It is designed to help you make informed decisions before committing serious enterprise investment.
With 86% of healthcare organizations already using AI, enterprises modernizing medical device software gain faster approvals, stronger differentiation, and long-term digital health advantage.
Why Are Enterprises Investing in Medical Device Software Development Now?
Enterprise healthcare transformation does not arrive with a single bold announcement. It shows up quietly. A product team is struggling to connect device data with hospital systems. A compliance lead preparing for a new audit cycle.
A boardroom conversation about unlocking new revenue beyond hardware sales. These are the moments driving today’s shift toward software-first medical devices.
This shift is backed by market reality. The global digital healthcare market is projected to reach $549.7 billion by 2028, fueled by connected care, intelligent diagnostics, and data-driven treatment models.
Enterprises investing in medical device software development now are positioning themselves to compete in a healthcare ecosystem where software defines differentiation.
What is pushing enterprises to invest now
- Healthcare delivery is moving beyond hospital walls: IoT-enabled connected treatment pathways, such as remote patient monitoring and virtual care programs, are becoming standard practice. Software development for medical devices now prioritizes secure data transmission, clinician dashboards, and reliable operation in home environments.
- Regulators are adapting to software-driven products: Clearer guidance for Software as a Medical Device, continuous software updates, and post-market monitoring expectations are shaping how products are built. Enterprises investing in structured medical instrument software development are navigating approvals with fewer late-stage surprises.
- Data integration has become non-negotiable: Providers expect devices to connect with EHR systems, support HL7 FHIR standards, and feed real-time clinical data pipelines. Medical device development now requires cloud-readiness and interoperability planning from day one.
- Smart healthcare technology in healthcare: AI-assisted diagnostics, adaptive therapy engines, and predictive maintenance features influence buying decisions. Software design for medical devices directly affects clinical confidence and brand trust.
- R&D teams are under pressure to extend product value: Software-driven upgrades, cybersecurity patching, analytics services, and digital companion apps are creating long-term revenue models beyond initial device sales.
The importance of medical device apparatus development is clear: it is no longer a supporting function but has become the foundation for scalable innovation, regulatory resilience, and sustainable enterprise growth.
What Types of Medical Device Software Should Enterprises Build?
Most enterprise product leaders face this decision earlier than expected. A roadmap meeting runs long. Someone asks whether the next product upgrade needs smarter hardware or smarter software. That question often sets the investment strategy for the next two years.
Medical device software development generally takes three directions within broader healthcare software categories. Each one comes with its own realities in medical device software design, compliance expectations, and commercial upside.
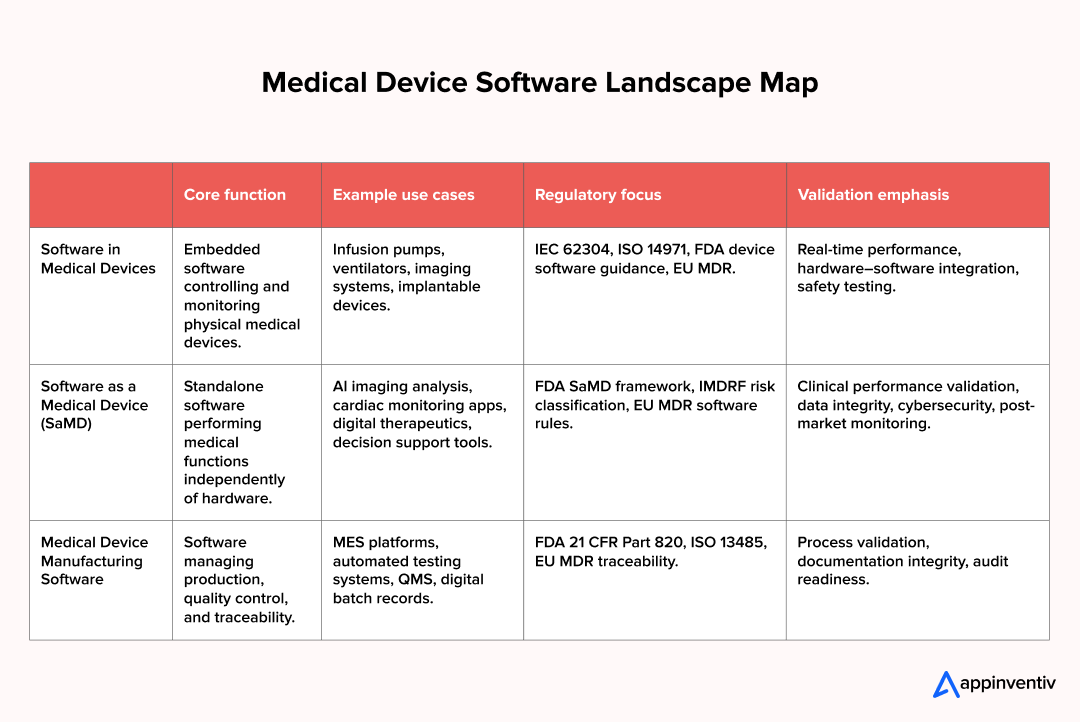
Software as a Medical Device is emerging as a major growth category, with the global SaMD market projected to reach USD 6.87 billion by 2032. Enterprises that enter this space early gain experience in regulated software delivery and in clinical data-driven innovation.
- Software inside a medical device is the logic that keeps equipment safe and predictable. Infusion pumps regulate dosage. Imaging systems process sensor data. Ventilators manage airflow cycles. In this category, software development for medical devices focuses on embedded control systems, real-time responsiveness, fault tolerance, and tight hardware integration. Safety engineering drives every design choice.
- Software as a Medical Device (SaMD) requires different approaches to medical device software design. The software itself performs the medical function. Examples of software as a medical device include next-generation healthcare applications that analyze cardiac rhythms, cloud platforms that flag anomalies in radiology scans, and digital therapeutics that enable personalized healthcare experiences and adapt treatment plans over time. Software as a medical device development demands robust data pipelines, clinical evidence generation, cybersecurity safeguards, and continuous performance monitoring post-launch.
- Medical device manufacturing software sits behind the scenes. These systems manage production traceability, automated testing, calibration workflows, and quality documentation. They matter because regulators now expect end-to-end visibility from design through manufacturing and post-market support.
Medical device software development applications span AI imaging interpretation tools, remote patient monitoring platforms, and decision support systems used in clinical workflows.
Interoperability across the Internet of Medical Things (IoMT) ecosystem is often where medical device software strategies succeed or stall. Connecting data from multiple devices, standardizing formats, and ensuring real-time visibility across care environments is complex in practice. Enterprises that solve this early gain faster integrations, stronger clinical adoption, and scalable digital health ecosystems.
See how we integrated 200+ health devices into a unified data platform built for real-world clinical environments.
As medical device ecosystems grow more connected, enterprises must plan interoperability, data governance, and integration readiness from the earliest design stages.
What Is the Difference Between Software in Medical Devices and Software as a Medical Device (SaMD)?
At a high level, one controls hardware, and the other delivers clinical intelligence. In software development for medical devices, this distinction shapes the architecture, documentation strategy, depth of validation, and long-term maintenance planning.
Embedded software within a device is designed to ensure deterministic behavior and hardware safety controls. SaMD systems are designed around data integrity, algorithm accuracy, and clinical risk management. That difference changes medical device software design, testing, release, and update strategies.
| Aspect | Software in Medical Device | Software as a Medical Device (SaMD) |
|---|---|---|
| Definition | Embedded software operating a physical medical device | Standalone software delivering a medical function |
| Regulatory pathway | Approved as part of the physical device | Approved as an independent medical product |
| Risk classification | Based on the hardware safety impact | Based on the clinical decision impact |
| Development lifecycle focus | Real-time control, hardware integration | Data processing, algorithm performance, cloud readiness |
| Validation requirements | Safety and hardware integration testing | Clinical validation and post-market monitoring |
How Does the Medical Device Software Development Process Work?
Enterprise leaders rarely struggle with vision. The real challenge appears once execution begins. Building software in regulated healthcare environments requires structure, traceability, and risk control at every stage. A mature medical device software development process brings those disciplines together so innovation does not collide with compliance.
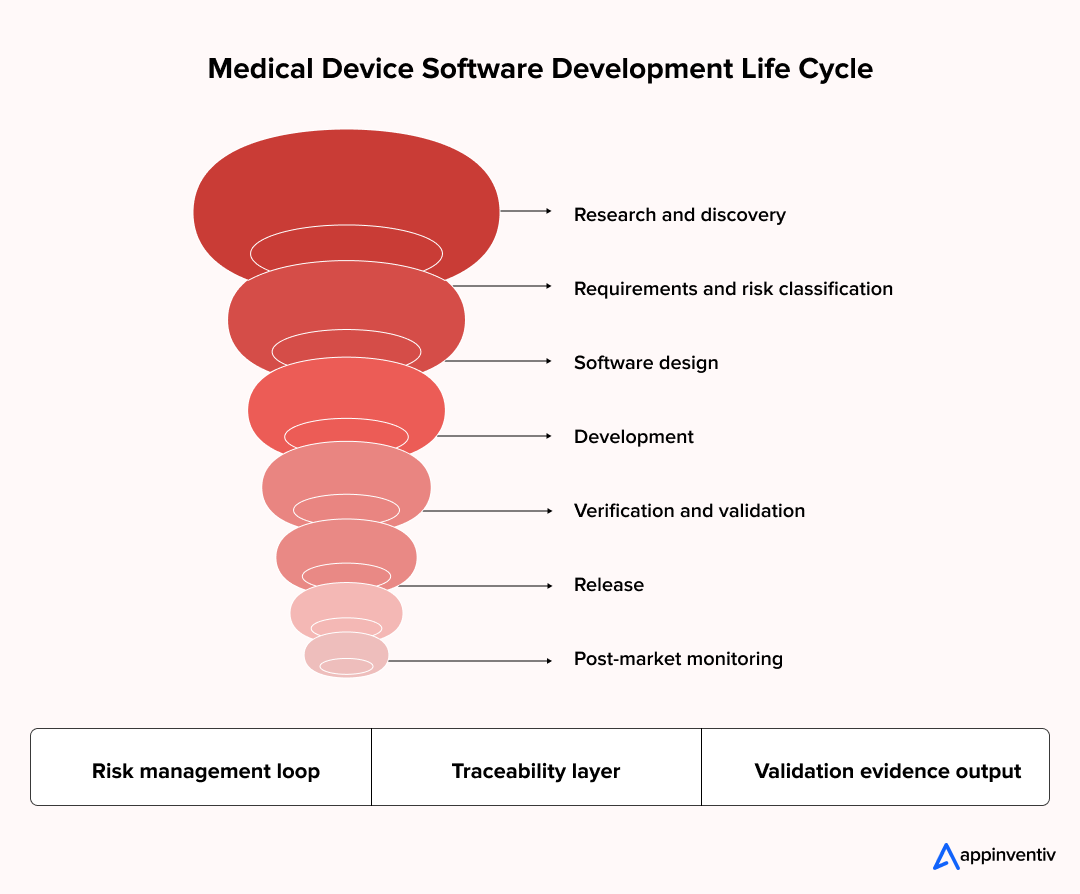
Below is how a robust medical device apparatus development life cycle runs inside healthcare software product development programs.
Step 1: Research and Discovery
Every successful program starts with clarity on clinical intent and patient impact. Teams analyze intended use, clinical workflows, competitive products, and regulatory classification. This early phase of medical device software research and development determines whether the solution will be embedded software, Software as a Medical Device, or part of a connected care platform.
Architecture direction, data strategy, and compliance planning are established before technical work begins.
Step 2: Requirements and Risk Classification
Business objectives now become system requirements and user needs. Risk management activities identify potential hazards, severity levels, probability of harm, and mitigation measures. These artifacts form the backbone of traceability, linking requirements, risks, test cases, and validation evidence throughout the project lifecycle.
Step 3: Medical Device Software Design
Technical blueprints take shape at this stage. Teams define system architecture, data flow models, interface specifications, and interoperability standards such as HL7 FHIR. Cybersecurity controls, access management, audit logging, and cloud or edge deployment decisions are locked in. Design reviews align engineering output with quality and regulatory expectations before development begins.
Step 4: Development and Implementation
Software development for medical devices occurs under a quality management system. Secure coding practices, version control, configuration management, and automated test pipelines are established. Continuous integration environments ensure that every build remains traceable, reviewable, and audit-ready. This phase focuses on stability, maintainability, and controlled change management.
Step 5: Verification and Validation
This phase produces the evidence that regulators and enterprise quality teams require. Unit testing validates individual components. Integration testing confirms system interactions. System verification checks functional and performance requirements. Usability validation ensures safe operation in clinical environments.
For Software as a Medical Device, clinical performance evaluation and algorithm verification are also conducted. Every result remains traceable to requirements and risk controls, ready for submission and audit review.
Step 6: Release and Controlled Deployment
Release follows formal change control procedures. Installation packages, release documentation, training materials, and operational guides are finalized. Enterprises define deployment validation steps, rollback procedures, environment checks, and access governance. This ensures stable rollouts across hospitals, labs, and remote care environments.
Step 7: Post-Market Monitoring and Lifecycle Support
After launch, continuous oversight begins. Teams monitor software performance, incident reports, cybersecurity vulnerabilities, and user feedback. Patch management, version updates, and corrective actions follow regulatory reporting pathways. For SaMD products, real-world performance data collection often continues throughout the authorization period. This phase protects long-term compliance, patient safety, and enterprise brand trust.
Post-launch responsibilities supported by managed IT services in healthcare define long-term product success. Continuous monitoring, secure handling of patient data, and compliant update cycles are essential once medical software enters real clinical use. Enterprises that operationalize this phase early maintain regulatory confidence and patient trust.
Explore how we built a secure, compliance-ready diabetes management platform supporting continuous patient monitoring.
Strong post-market frameworks turn medical software from a one-time release into a trusted, evolving clinical asset.
Which Medical Device Software Standards and Regulations Must Enterprises Follow?
Most compliance discussions start in a meeting that runs five minutes over. A product owner wants to lock timelines. A regulatory lead asks when documentation will begin. Someone realizes compliance cannot be “handled later.” In medical device software development, it is part of the build from the very first sprint.
Enterprises usually anchor their software programs around a few core standards.
- IEC 62304 shapes how software is created and maintained. It guides planning, design, coding practices, testing discipline, release controls, and issue management. It also introduces safety levels that quietly influence how deep your testing, traceability, and documentation need to go.
- ISO 14971 handles risk. Teams use it to identify potential hazards, assess patient impact, define safeguards, and track whether the remaining risk is acceptable. It connects engineering decisions to safety evidence that auditors expect to see.
Then come market-specific requirements.
- For the US, FDA guidance for Software as a Medical Device focuses on clinical performance, algorithm behavior, cybersecurity posture, and how software updates are governed after launch.
- For Europe, the EU MDR defines software classification, clinical evaluation expectations, and post-market surveillance responsibilities.
One more standard often shows up late if teams are not careful. IEC 62366 addresses usability engineering. It ensures that clinicians and patients can operate the software safely in real-world environments, not just in test labs.
A regulatory shift is also arriving in the US. As of February 2, 2026, the FDA has transitioned to the Quality Management System Regulation (QMSR), aligning device software quality requirements directly with ISO 13485.
For enterprises, this reduces framework duplication but raises expectations around design controls, traceability, and audit readiness. Teams that prepare early for QMSR alignment will move through future FDA inspections with fewer process changes and lower compliance risk.
Our regulated delivery teams help enterprises design, build, and validate medical software in accordance with IEC 62304 and global compliance frameworks.
Why Risk Class Changes Everything?
Risk classification sets the workload behind the scenes. Higher-risk medical device software design demands deeper validation, stronger clinical evidence, stricter change control, and continuous monitoring after release. Lower-risk products follow lighter paths, though controlled processes still apply.
Enterprises that align these requirements early avoid last-minute compliance fire drills and move through approvals with confidence.
How Much Does Medical Device Software Development Cost for Enterprises?
Once a product idea gets executive approval, the next question is always the same. How much will this actually take to build? In medical equipment software development, cost is shaped less by coding and more by compliance, validation, and long-term support.
Most software development cost for medical devices falls within a clear enterprise range.
Typical Enterprise Cost Range
| Project Scope | Investment Range (USD) | Typical Inclusions |
|---|---|---|
| Lower complexity and lower-risk software | $50K to $150K | Core features, foundational compliance documentation, and basic testing |
| Moderate complexity and medium-risk software | $150K to $300K | Full lifecycle development, risk management, validation testing, and system integrations |
| High complexity or Software as a Medical Device platforms | $300K to $500K + | Advanced architecture, clinical validation, cybersecurity hardening, regulatory submission readiness, and post-market monitoring setup |
These figures cover initial development. Ongoing updates, security maintenance, and compliance reporting extend investment across the product lifecycle.
What Drives Medical Device Software Development Costs?
Cost drivers often appear in day-to-day project realities. A validation plan expands. A cybersecurity review adds new controls. A hospital integration request comes late in testing. These moments shape the final budget.
Key drivers include:
- Post-market surveillance and lifecycle costs: Continuous monitoring, cybersecurity patching, regulatory reporting, and controlled updates extend the total cost of ownership beyond initial development.
- Software category: Embedded software, Software as a Medical Device, and medical device manufacturing software each require different architectures and validation approaches.
- Risk classification level: Higher-risk software demands deeper verification, stronger clinical evidence, and stricter documentation control.
- Regulatory and compliance workload: IEC 62304 alignment, ISO 14971 risk activities, usability engineering, and audit-ready traceability add significant effort.
- Technology and integration scope: Cloud infrastructure, HL7 FHIR interoperability, data security layers, and analytics pipelines influence the depth of engineering.
- Post-market obligations: Performance monitoring, cybersecurity patching, defect management, and regulatory reporting continue after release.
Enterprises that account for these factors early avoid financial surprises and keep delivery timelines steady.
What Are the Biggest Challenges in Software Development for Medical Devices?
Every enterprise program reaches a point where planning decks stop helping. A pilot deployment is scheduled. A regulatory review is booked. A hospital integration test is about to begin. That is when the real challenges in software development for medical devices start to surface.
Some are technical. Some are process-driven. Others are rooted in organizational maturity. Here is where enterprises most often feel pressure.
Regulatory Execution
Compliance is rarely static. Requirements evolve. Validation evidence expands. Audit questions appear close to submission timelines. Medical equipment software development demands continuous alignment between engineering, quality, and regulatory teams.
What this means in practice:
- Documentation and traceability must stay current
- Validation plans need frequent refinement
- Late regulatory feedback can impact release schedules
Legacy Integration
Most enterprises build on existing technology stacks. Software development for medical devices must connect with device firmware, hospital IT systems, and data platforms.
Common friction points:
- Older systems lacking modern APIs
- Custom hospital interfaces
- Firmware limitations discovered during integration testing
Also Read: Exploring the Role of Healthcare APIs as a Strategic Business Asset for Competitive Advantage
Interoperability
Healthcare ecosystems expect seamless data exchange. EHR connectivity, HL7 FHIR pipelines, clinician dashboards, and analytics platforms must all receive reliable data.
Where issues arise:
- Inconsistent data formats
- Interface instability under load
- Delayed discovery of workflow mismatches
Also Read: HL7 Integration for Healthcare Businesses: Exploring 10 Benefits and Use Cases
Cybersecurity and Data Privacy
Security expectations are rising across global regulators. Encryption, access control, vulnerability testing, and secure update mechanisms are now baseline requirements.
Typical healthcare cybersecurity challenges include:
- Expanding security review cycles
- Remediation of penetration test findings
- Maintaining compliance during software updates
Also Read: Navigating Healthcare Data Security: Common Challenges and Proven Best Practices
Organizational Readiness
Process maturity matters as much as engineering skill. Teams must adopt documentation discipline, traceability practices, and post-market monitoring routines.
Signs of readiness gaps:
- Inconsistent quality processes
- Limited regulatory experience in product teams
- Reactive compliance efforts
Should Enterprises Build, Buy, or Partner for Medical Device Software Development?
This decision typically arises once leadership commits to a software-driven device strategy. A steering committee meets. Engineering wants control. Compliance wants predictability. Finance wants clarity. The choice between building, buying, or partnering shapes cost, timelines, and regulatory exposure from the start.
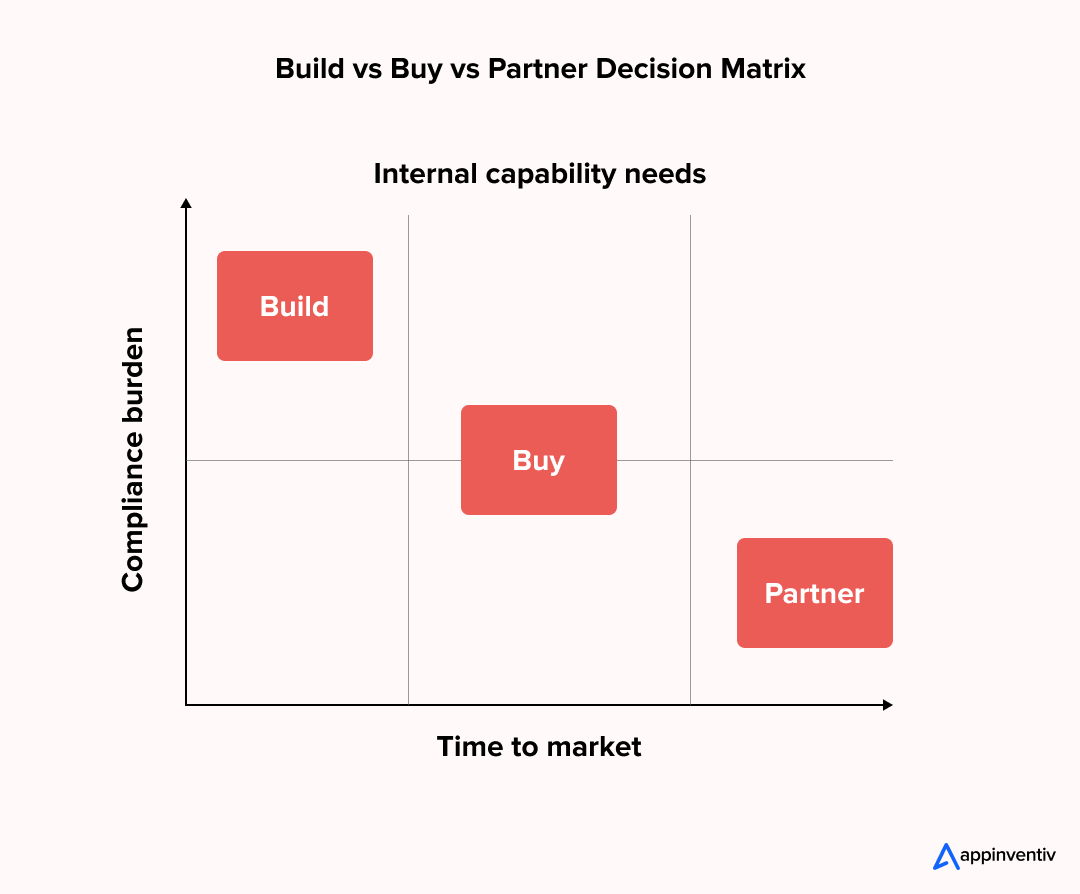
Here is how large enterprises typically evaluate their options.
Build vs Buy vs Partner Comparison
| Approach | What It Means | Where It Works Best | Common Limitations |
|---|---|---|---|
| Build in-house | Internal teams manage full medical tool software development | Enterprises with mature quality systems, regulatory expertise, and strong engineering capacity | High upfront investment, longer delivery cycles, and internal compliance load |
| Buy off-the-shelf | Ready-made software platforms or licensed solutions | Standardized workflows with limited customization needs | Limited differentiation, integration constraints, vendor dependency |
| Partner with a specialist | External team provides custom medical device software development services within your compliance framework | Enterprises seeking faster execution and regulated software expertise | Requires governance alignment and vendor coordination |
Partnering does require governance and oversight. Still, the right partner reduces effort instead of adding complexity. A custom medical device software development firm like Appinventiv works within your quality frameworks, maintains a disciplined approach to traceability, and brings proven experience in regulated delivery. This helps enterprises move faster without losing control or audit readiness.
Decision Criteria for Large Enterprises
The right model depends on how ready your organization is to assume responsibility for regulated software at scale.
Key factors usually include:
- Internal quality and regulatory maturity
- Engineering capacity and domain experience
- Custom medical device software development and product differentiation goals
- Time-to-market pressure
- Long-term ownership strategy
A clear view of these factors prevents stalled programs and late compliance surprises.
We help enterprises assess internal readiness, compliance maturity, and sourcing strategy before committing major medical software investments.
How to Choose the Right Medical Device Software Development Partner?
This choice usually gets made in a high-stakes moment. A shortlist is ready. Timelines are tight. Someone asks a simple question. Can this partner handle compliance as confidently as they handle code? In medical tool software development, that answer determines whether your program accelerates or stalls.
For enterprise teams, the right partner should reduce regulatory pressure, not increase it. Here is a practical evaluation checklist used by large organizations.
Enterprise Vendor Evaluation Checklist
- IEC 62304 delivery maturity: Proven experience running structured software lifecycle processes, safety classification handling, traceability management, and controlled change workflows.
- Quality management systems: Ability to work inside your QMS, maintain documentation discipline, and produce audit-ready development and validation evidence.
- Verification and validation expertise: Hands-on capability in system verification, usability validation, clinical performance testing, and requirement-to-risk test mapping.
- Cybersecurity practices: Secure medical device software design, vulnerability management routines, penetration testing, and controlled software update mechanisms.
- Global deployment readiness: Familiarity with FDA and EU MDR pathways, data privacy rules, localization needs, and multi-region release management.
A partner that meets these criteria integrates smoothly into your governance structure and helps you deliver compliant, scalable medical software with confidence.
What Future Trends Are Shaping Medical Device Software Development?
If you look at enterprise product roadmaps today, a pattern appears. More software updates. More data pipelines. More connected devices in real-world care environments. Medical software development is shifting from static releases to living digital ecosystems.
A few trends are driving this shift.
- AI-powered Software as a Medical Device is moving from experimentation to regulated production. Imaging analysis tools, clinical risk engines, and adaptive therapy platforms now require clinical evidence, algorithm governance, and real-world performance tracking from day one.
- Cloud-native and edge-connected architectures are becoming the default. Critical processing happens on-device for speed and safety, while heavy analytics and model updates run in secure cloud environments. This balance improves reliability, scalability, and remote care support.
- Continuous validation models are gaining regulatory acceptance. Instead of freezing software after approval, enterprises now build controlled pipelines for ongoing verification, cybersecurity patching, and monitored performance updates.
- Interoperable healthcare ecosystems are no longer optional. Devices must integrate with EHR platforms, analytics systems, and care coordination tools through HL7 FHIR and standardized APIs.
- EU AI Act obligations for high-risk healthcare AI begin in August 2026. Enterprises building AI-powered SaMD must ensure algorithm transparency, data governance, and bias monitoring. SaMD development is now extending beyond performance into continuous AI compliance management.
Enterprises that adapt to these trends build medical software that evolves with clinical and regulatory expectations, not behind them.
Also Read: Top Healthcare Trends That Will Redefine The Industry in 2025
Why Is Appinventiv the Right Partner for Medical Device Software Development?
At some point, every enterprise team asks the same question. Who can build this without slowing us down or putting compliance at risk? That decision shapes timelines, approvals, and long-term product credibility.
As a leading medical software development services company, Appinventiv brings deep experience in delivering regulated healthcare software. The focus is not only on building technology, but on delivering compliant, scalable, and audit-ready medical solutions.
Our healthcare delivery footprint
- 500+ digital health platforms delivered
- 450+ healthcare clients served
- 10+ years in HealthTech project execution
- 300+ connected medical devices integrated
What this means for enterprise programs
- 99.90% uptime across critical healthcare systems
- 45% operational efficiency gains in hospital environments
- 90%+ clinical data accuracy in deployed platforms
- 95% patient satisfaction in patient-facing applications
How we work
- IEC 62304-aligned software lifecycle execution
- Risk and traceability are built into every sprint
- Validation and documentation kept audit-ready
- Security-first architecture and controlled updates
If your enterprise is planning a medical device software initiative, a focused conversation can help define the fastest and safest path forward.
Frequently Asked Questions
Q1. What is software as a medical device (SaMD)?
A. Software as a Medical Device refers to standalone software that performs a medical function without being embedded in physical hardware. It can analyze clinical data, support diagnosis, recommend treatments, or monitor patient conditions. Because it directly influences medical decisions, SaMD is regulated as a medical device and requires defined validation and post-market performance monitoring.
Q2. What are the best practices in medical device software development?
A. Best practices include following IEC 62304 lifecycle processes, integrating ISO 14971 risk management, maintaining traceability from requirements to tests, applying secure coding standards, and generating audit-ready validation evidence. Enterprises also establish quality management systems, controlled change procedures, and post-market monitoring to maintain compliance throughout the software lifecycle.
Q3. What are the five phases of medical device software development?
A. The five core phases include research and requirement definition, risk classification and system design, software development and implementation, verification and validation testing, and release with post-market monitoring. Each phase produces traceable documentation and evidence to support regulatory review, safety assurance, and long-term compliance.
Q4. How much does it cost to build software for medical devices?
A. Enterprise medical software development typically costs between 50,000 and 500,000 USD. Investment levels depend on software complexity, risk classification, compliance workload, validation depth, cybersecurity needs, and post-launch monitoring requirements. Higher-risk or SaMD platforms usually require larger budgets due to clinical evidence and regulatory preparation.
Q5. How long does it take to create medical device software?
A. Most enterprise-grade medical device software projects take between 6 and 12 months. Timelines vary based on regulatory classification, validation scope, integration complexity, and approval cycles. SaMD products or high-risk systems may take longer due to clinical performance evaluation and extended regulatory review stages.
Q6. What regulatory standards apply to medical device software?
A. Medical device software must align with IEC 62304 for software lifecycle management, ISO 14971 for risk management, IEC 62366 for usability engineering, FDA SaMD guidance for US market entry, and EU MDR for European compliance. Together, these frameworks define safety, validation, documentation, and post-market responsibilities.
Q7. How do enterprises validate and ensure compliance in medical device software?
A. Enterprises validate software through unit, integration, system, usability, and clinical performance testing. They maintain traceability from requirements to risk controls and validation evidence. Compliance is ensured through quality management systems, controlled change processes, audit-ready documentation, cybersecurity monitoring, and structured post-market surveillance activities.


- In just 2 mins you will get a response
- Your idea is 100% protected by our Non Disclosure Agreement.
How to Build a Custom Pediatric EMR and EHR System?
Key takeaways: Clinical Precision: Custom systems accommodate pediatric-specific data points like percentile curves and weight-based longitudinal dosing. Interoperability: Seamless data exchange via HL7 FHIR ensures your practice stays connected to pharmacies, labs, and state registries. Regulatory Resilience: Built-in compliance with HIPAA, HITECH, and MACRA/MIPS reduces legal friction. Enhanced Engagement: Parent portals reduce administrative overhead by…

Change Management in Healthcare: Principles, Processes, and Models
Key Takeaways Change in healthcare fails quietly when ownership, workflow alignment, and follow-through are missing. Successful change management in healthcare focuses on adoption, not just system implementation. Clinical workflows and workforce capacity determine whether transformation sticks or stalls. Governance, clear accountability, and post-go-live support matter more than the model used. Sustainable healthcare transformation depends on…
A Practical Guide to Building Your Mental Health Chatbot - Use Cases, Cost, & ROI
Key takeaways: Mental health chatbots work when they know their limits. They’re most useful as a gentle first step, not as a stand-in for real care. Good chatbot design is more about judgment than AI. Clear boundaries, calm responses, and safety matter more than smart language models. Enterprises invest in chatbots to make support easier…