- Why is it Important to Develop Compliant Healthtech Apps in the UK
- Key Regulations and Standards in UK Healthcare
- UK Medical Device Regulations (UK MDR 2002)
- Digital Technology Assessment Criteria (DTAC)
- General Data Protection Regulation (GDPR)
- Health and Social Care Act 2012
- Data Protection and Security Toolkit (DSPT)
- NHS Code of Conduct for Data-Driven Health and Care Technology
- ISO Standards (ISO 13485 and ISO 27001)
- MHRA Guidance
- Navigating MHRA Compliance Guidelines
- UKCA Marking
- Safety and Performance
- Clinical Evaluation
- Post-Market Surveillance
- Technical Documentation
- Risk Management
- Aligning with NHS Digital Standards
- Digital Technology Assessment Criteria (DTAC)
- Clinical Safety
- Data Protection
- Technical Assurance
- Interoperability
- Usability, Accessibility, and AI-Driven Support
- Evidence-Based Practices Framework (EBPF)
- Pillars of UK Healthtech App Compliance
- Governance and Oversight
- Policies and Procedures
- Risk Management Framework
- Training and Awareness
- Monitoring and Auditing
- Reporting and Corrective Action
- Continuous Improvement
- Critical Challenges and Practical Solutions in UK Healthcare Compliance
- 1. Regulatory Complexity
- 2. Clinical Validation and Evidence Generation
- 3. Data Security and Data Protection
- 4. NHS Systems Interoperability
- 5. Resource Constraints
- 6. Being Accessible and Inclusive
- How to Handle False or Misleading Claims in Healthtech App Marketing
- Trust Appinventiv to Align Your HealthTech App with Regulatory Standards
- FAQs
Key takeaways:
- To be safe, secure, and legally compliant, healthtech apps in the UK must comply with the NHS, MHRA, and GDPR.
- The initial implementation of compliance, including data protection, clinical safety, and technical standards, facilitates approval and user confidence.
- It is crucial to align with such frameworks as DTAC, DSPT, and ISO standards to achieve NHS adoption and succeed over time.
- Strategic compliance is not only a way to prevent legal risks but also contributes to a broader following and trust within the UK healthtech market.
The UK is rapidly turning into a health technology center, and digital solutions are increasingly contributing to patient care, clinical operations, and efficiency. Healthtech innovation, in this case, does not only mean developing the latest apps but also providing solutions that are secure, reliable, and trusted throughout the healthcare system.
However, there is a complex regulatory environment to contend with to build apps in this space. The NHS has high standards of clinical safety and patient outcomes, and the MHRA has strict medical device regulations to ensure efficacy and compliance. Healthcare businesses should take into account data protection, clinical validation, and patient safety from the very first design phases to ensure UK healthtech app compliance adherence.
Winning in the UK healthtech market is possible by converting these regulatory requirements into strategic benefits, as demonstrated by digital-first healthcare solutions for the NHS that prioritize compliance from day one. Compliant apps gain the trust of clinicians, regulators, and patients, leaving potential opportunities for greater adoption and sustained influence.
Touching the ground-based NHS and MHRA guidelines in the development allows the companies not only to comply with the legal requirements but also to establish their solutions as reliable, ground-breaking instruments in healthcare.
In this blog, we will discuss why it is important to build compliant healthtech apps in the UK, key regulations that need to be followed, the role of MHRA and NHS guidelines, pillars of UK healthtech compliance, critical challenges with solutions, and how to handle false compliance-related claims. Let’s dig further.
Strengthen trust and market access by aligning your healthtech app with full compliance and patient data protection
Why is it Important to Develop Compliant Healthtech Apps in the UK
Developing a compliant healthtech application is not only a regulatory requirement but also a decisive business decision in the highly regulated UK healthcare system. Conformity ensures that all digital health solutions are national standards, patient-sensitive, and trusted by healthcare providers, investors, and end users.
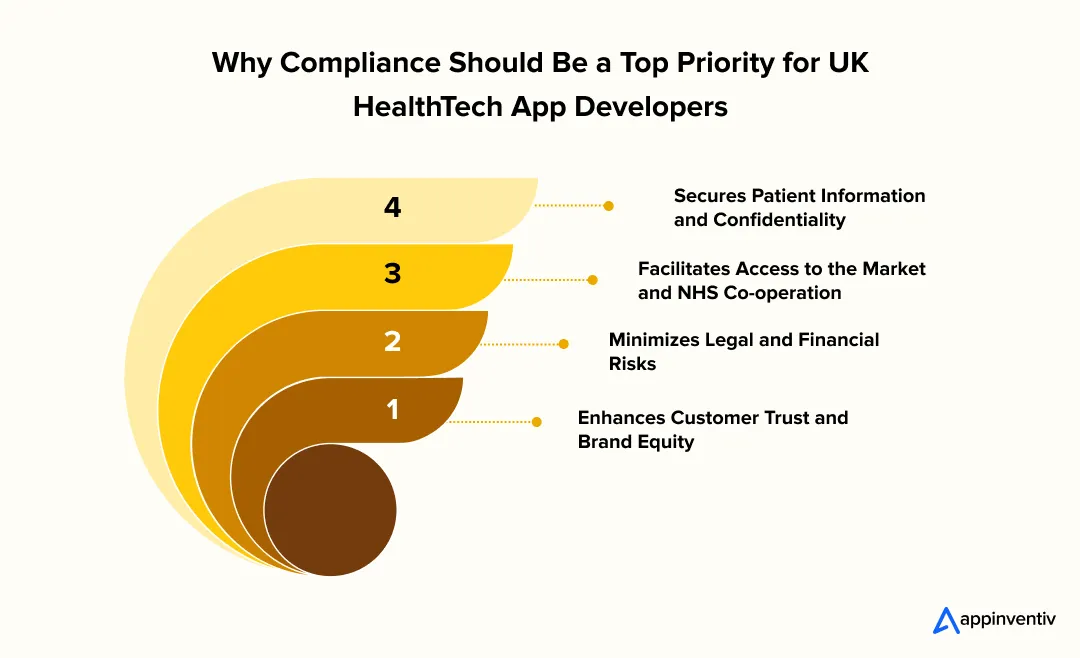
Secures Patient Information and Confidentiality: Compliance with UK-based regulations such as the Data Protection Act, NHS DSPT, and Cyber Essentials prevents data breaches and enhances the app’s effectiveness in handling sensitive health data.
Facilitates Access to the Market and NHS Co-operation: Integrating with NHS systems or obtaining permission to use them in clinical settings often requires acceptance of DCB0129, DTAC, and other NHS-aligned standards. Even innovative solutions can have barriers to adoption without satisfying those standards.
Minimizes Legal and Financial Risks: Failure to comply may lead to severe fines, damage to reputation, or being kicked out of the digital marketplace. Regulatory alignment guarantees continuity and operational believability in the business.
Enhances Customer Trust and Brand Equity: It is more likely that patients and practitioners will interact with a product that is ethically responsible and transparent. Adherence is a credibility marker that will distinguish your app within the competitive environment.
Key Regulations and Standards in UK Healthcare
Navigating the UK healthcare sector requires strict adherence to established regulations and standards. These frameworks ensure patient safety, data security, and the delivery of reliable digital health solutions. Understanding and implementing them is essential for any successful healthtech app.

UK Medical Device Regulations (UK MDR 2002)
UK MDR 2002 regulates medical devices, including software that is a medical device (SaMD). In the case of any healthtech app UK, these regulations lead to clinical safety, risk management, and documentation.
Before entering the market, businesses have to undertake conformity evaluation and clinical analysis. Safety standards also need to be kept through post-market surveillance. MDR compliance early in the design of your app makes your solution a UK healthtech app compliance standard.
Digital Technology Assessment Criteria (DTAC)
DTAC is the NHS model of assessing digital health technologies. It includes clinical safety, technical security, interoperability, usability, and data protection. Any digital health app assessment in the UK must meet DTAC standards so that the apps can be adopted safely across NHS services.
By complying with these requirements, your app would become a healthcare app UK that is compliant with functional implementation.
General Data Protection Regulation (GDPR)
GDPR establishes the principles of managing personal and patient information. Data protection for health apps UK, involves secure storage, informed consent, transparency, and close data sharing management.
Adherence prevents legal fines, as well as fosters trust between patients and clinicians. All healthtech applications should incorporate GDPR requirements from the earliest stages of development.
Also Read: Appinventiv’s GDPR-Ready HealthTech Solutions
Health and Social Care Act 2012
In this Act, NHS Digital has the power to provide health IT regulation and digital integration. In the case of any healthtech app UK, compliance will guarantee adherence to NHS governance, operational standards, and data handling protocols. It is a guiding principle that promotes safe and standardized adoption in the NHS ecosystem.
Data Protection and Security Toolkit (DSPT)
NHS Digital requires organizations that access patient information to certify them under DSPT. DSPT completion is an indication that your compliant healthcare app UK is in accordance with the national standards of information security. It also checks the technical security, access controls, and privacy controls, and ensures that the stakeholders that the organization is dedicated to patient safety.
NHS Code of Conduct for Data-Driven Health and Care Technology
The code offers ethical and practical principles for implementing digital solutions in healthcare. Subsequently, this will keep your healthtech app UK in line with the requirements of clinical safety, governance, and accountability. This is enhanced by adherence and contributes to NHS adoption.
ISO Standards (ISO 13485 and ISO 27001)
ISO 13485 sets quality management systems for medical devices, whereas ISO 27001 focuses on information security. These standards for a health tech application in the UK will ensure operational reliability, data security, and compliance with regulations. They are combined to create a strong basis for the UK healthtech app compliance.
MHRA Guidance
The MHRA offers software classification regulation, a regulatory pathway, and clinical safety. With such guidance, your compliant healthcare app UK will be well-regulated in terms of legal and safety requirements. Early alignment is risk-minimizing to regulators and speeds up approval of NHS deployment.
Navigating MHRA Compliance Guidelines
For businesses pursuing NHS-approved app development, compliance with the Medicines and Healthcare products Regulatory Agency (MHRA) is a fundamental requirement. The MHRA regulates the classification, testing, and monitoring of medical software in order to guarantee patient safety and the reliability of products.
The UK medical software classification can cover any healthtech app UK that has a diagnostic, monitoring, or informational aspect of clinical care, and it cannot be put on the market without formal assessment and certification.
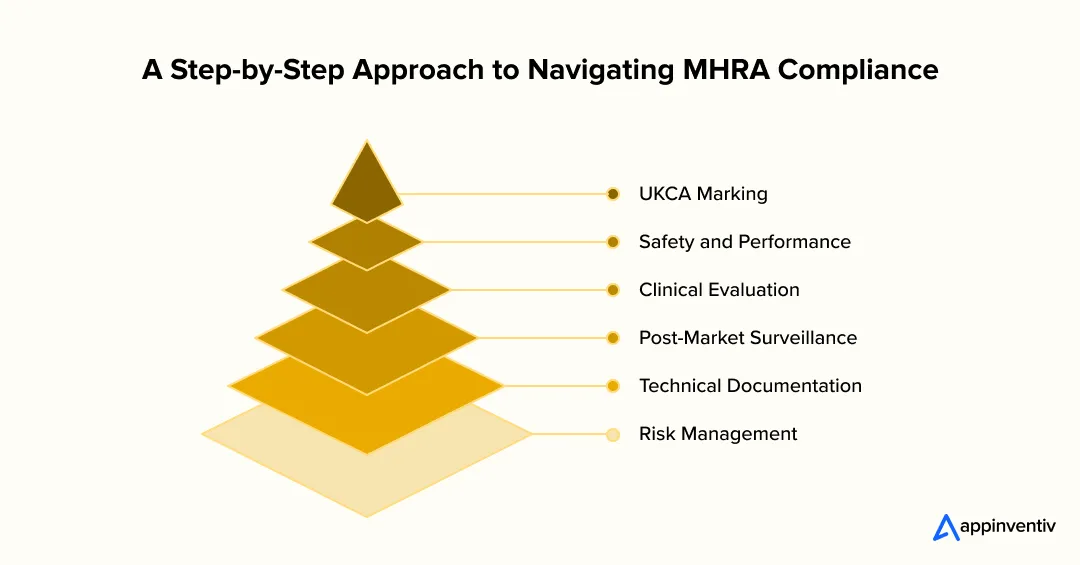
UKCA Marking
The UK Conformity Assessed (UKCA) mark is a compulsory mark for medical devices and software products sold in Great Britain. It certifies the fact that a product is safe and has acceptable performance in accordance with national standards.
Depending on classification, the businesses might require independent evaluation by an approved body. Obtaining this certification will provide a clear indication of complete MHRA compliance app UK and a major step towards acceptance by the NHS.
Safety and Performance
Under MHRA regulations, the developer must demonstrate that their apps are safe, reliable, and work under all the expected conditions. Testing should confirm performance in relation to clinical claims and identification and control of possible risks. Meeting these standards strengthens UK healthtech app compliance and establishes trust among clinicians and patients.
Clinical Evaluation
Clinical assessment provides a written report that justifies the app’s purpose and its utility in medicine. This can be in terms of literature reviews, usability testing, or clinical trials. The MHRA anticipates that all the findings will be traceable, reproducible, and scientifically explained. The step will ensure that the app aligns with real-world healthcare requirements and can be safely deployed.
Post-Market Surveillance
The release must be followed by continuous monitoring to identify safety concerns and the evaluation of the performance. Businesses are required to keep the records of incidents and complaints, and corrective measures. Proper monitoring is an assurance that the product is a valid healthcare application UK all throughout its lifecycle and still matches the regulatory demands.
Technical Documentation
All businesses shall keep a technical file containing product specifications, test results, validation reports, and conformity statements. The file can be used as proof of compliance with the regulations and audit preparedness.
Risk Management
In line with the ISO 14971, businesses should come up with a structured risk management process. This involves determining the hazards, estimating and assessing the risks, and implementing the control measures. By providing strong safety and compliance in the long term, integrated risk management is a testament to the UK medical software classification framework.
These principles incorporated at the earliest stage of design will make certification easier, reduce compliance risk, and increase market credibility. An effective strategy for developing an MHRA-approved app has a well-documented approach at its core.
Appinventiv Insights
Begin by aligning your app with MHRA requirements by mapping the key clinical workflows. This will enable your team to focus on documentation, evidence gathering, and risk mitigation so that regulatory approvals become easier and costly iterations can be avoided in the future.
Aligning with NHS Digital Standards
To fully comply with UK healthtech apps, businesses should also meet the standards set by NHS Digital, which establishes criteria for safety, interoperability, and data security across the healthcare system. These standards will guarantee that every healthtech app UK has the chance to integrate smoothly into the NHS infrastructure through custom app development, as well as achieve patient privacy and clinical safety expectations.

Digital Technology Assessment Criteria (DTAC)
The NHS digital technology assessment framework assesses digital health solutions across five fundamental domains: clinical safety, data protection, technical assurance, interoperability, and usability. The initial stage toward official NHS adoption is usually the meeting of standards set by DTAC.
Clinical Safety
Apps have to be developed and tested to avoid harming the patients. Documentation is also part of compliance, according to the NHS Digital DCB0129 and DCB0160 standards, with a specific clinical safety officer.
Data Protection
The businesses will be required to adhere to the UK GDPR and NHS data management policies. Healthy data encryption, safe storage, and open consent procedures will guarantee compliance with the NHS health app guidelines and preserve patient confidence.
Technical Assurance
Technical assurance is the assessment of the security, reliability, and scalability of the app’s infrastructure. It guarantees that the solution can work within NHS networks and withstand the pressure of high usage.
Interoperability
The interoperability in the NHS systems relies on the smooth exchange of data. Applications should be open-standardized based on FHIR (Fast Healthcare Interoperability Resources) to be able to connect with electronic health records and already available clinical platforms. This is one of the fundamental requirements of NHS health app guidelines.
Also Read: How to Achieve Interoperability in Healthcare IT
Usability, Accessibility, and AI-Driven Support
The digital adoption of health apps depends heavily on user experience. NHS Digital expects apps to be accessible (including WCAG) and user-friendly for both patients and medical staff. With numerous AI-driven innovations in the UK, healthcare experts are now leveraging AI-backed features such as personalized guidance, predictive alerts, or workflow optimization, can enhance usability while maintaining compliance with NHS standards.
Evidence-Based Practices Framework (EBPF)
The ESF is a program developed by the National Institute for Health and Care Excellence (NICE) to evaluate digital health technologies in terms of clinical effectiveness and value. Adherence to such a framework can enable businesses to illustrate real health results and financial benefits within their NHS-approved app development strategy.
When businesses incorporate the NHS Digital standards and frameworks into design, testing, and governance, they can ensure their solutions meet high-quality standards, whether regulatory or operational. Such alignment not only enables deployment to NHS systems but also makes each solution a well-regarded, compliant healthcare app in the UK, with the ability to create an effective, long-term impact on the UK healthtech environment.
Pillars of UK Healthtech App Compliance
UK HealthTech app compliance is supported by a comprehensive framework that ensures every phase of development, including design, testing, and deployment, meets the highest clinical and regulatory standards. Compliance with healthtech apps in the UK is more than a legal requirement; it builds long-term trust among patients, clinicians, and regulators. Let’s have a look at the key pillars.
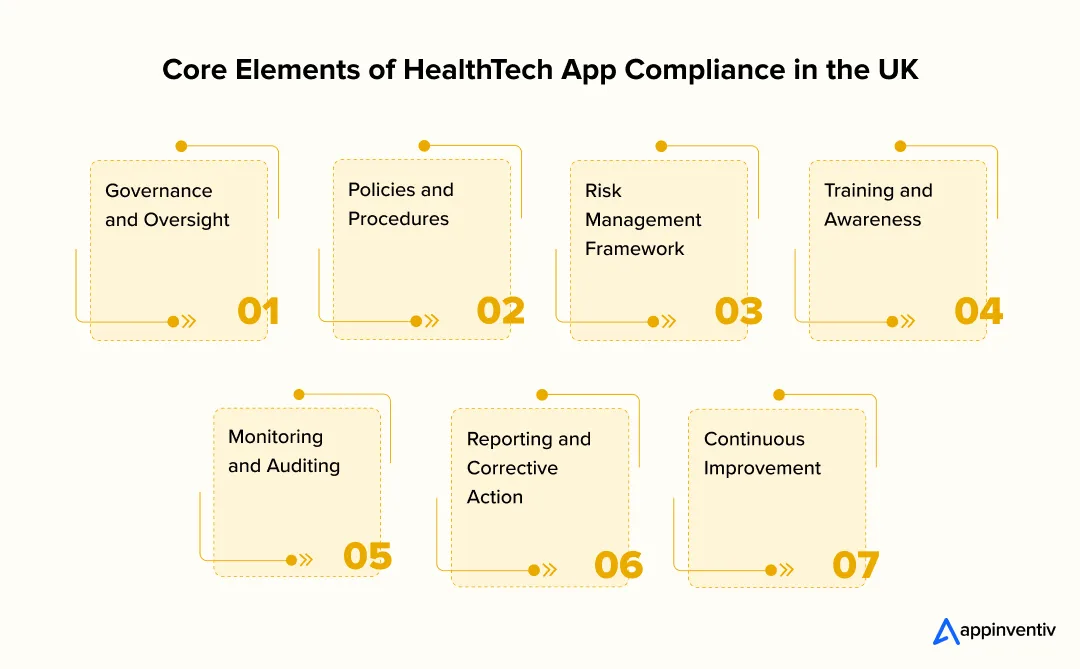
Governance and Oversight
Good governance brings accountability in leadership to the working teams. Compliance officers or special committees offer a good opportunity to ensure proper compliance with MHRA, NHS, and data protection guidelines. This form of governance strengthens transparency and makes every compliant healthcare app UK conform to regulatory objectives and clinical integrity.
Policies and Procedures
Any compliance strategy is based on documented policies. These policies must incorporate the direction of MHRA, NHS Digital, and GDPR requirements to ensure data protection for health apps UK. Written procedures would provide consistency in the application of safety, quality, and security practices within all departments of the app development.
Risk Management Framework
A robust risk management process is central to both digital health app assessment UK and overall safety assurance. It will be directed by ISO 14971 and will entail defining, assessing, and addressing the possible risks, technical, clinical, data-related, etc., across the product lifecycle. It is a proactive strategy that will identify vulnerabilities in early stages and ensure that it remains aligned with the UK healthtech apps compliance requirements.
Training and Awareness
A compliance program is only as effective as the people who implement it. Training will also be done regularly so that the teams know their role in regulation, whether it be in the security of the codes or in the confidentiality of the patient. In any healthtech app UK, the ethical issues of data handling, reporting requirements, and NHS Digital policies have to be considered in order to comply with them.
Monitoring and Auditing
Ongoing monitoring confirms the appropriateness of compliance practices and ensures that the app stays at the level of the regulatory standards. Planned audits, performance reviews, and post-market surveillance aid in monitoring the problems before they blow out of proportion. Evidence in the processes of digital health app assessment UK is also enhanced through regular evaluation as a sign of performance maturity and long-term reliability.
Reporting and Corrective Action
A proper reporting system will enable teams to record problems on time and implement corrective measures openly. It should incorporate controls for recording cases, alerting regulators when necessary, and ensuring cases are resolved. That kind of accountability would ensure the safety and effectiveness of a compliant healthcare app in the UK throughout its lifecycle.
Continuous Improvement
The compliance is not hard and fast, and it changes with the technology and regulations. Frequent changes of internal policies, technical documentation, and training modules would keep it in line with MHRA and NHS standards. The continuous review will protect data protection in health apps in the UK, keeping digital systems safe, ethical, and responsive to new issues.
Appinventiv Insights
Engage a systematized feedback loop of stakeholders, including patients, clinicians, and regulators, in the initial stages of the development cycle. Real-world input will ensure that the app is not only compliant with the regulations but also responds to actual issues related to usability and safety, making it more trusted and adopted in the long term in the UK healthtech ecosystem.
Critical Challenges and Practical Solutions in UK Healthcare Compliance
The development of digital health solutions in the UK is associated with its own challenges. The world of MHRA regulations, NHS Digital standards, and patient data requirements can be complicated, and innovations must be focused on ensuring that the affordability of compliance proceeds in parallel. These issues are important to ensure the UK’s digital health transformation is achieved strategically.
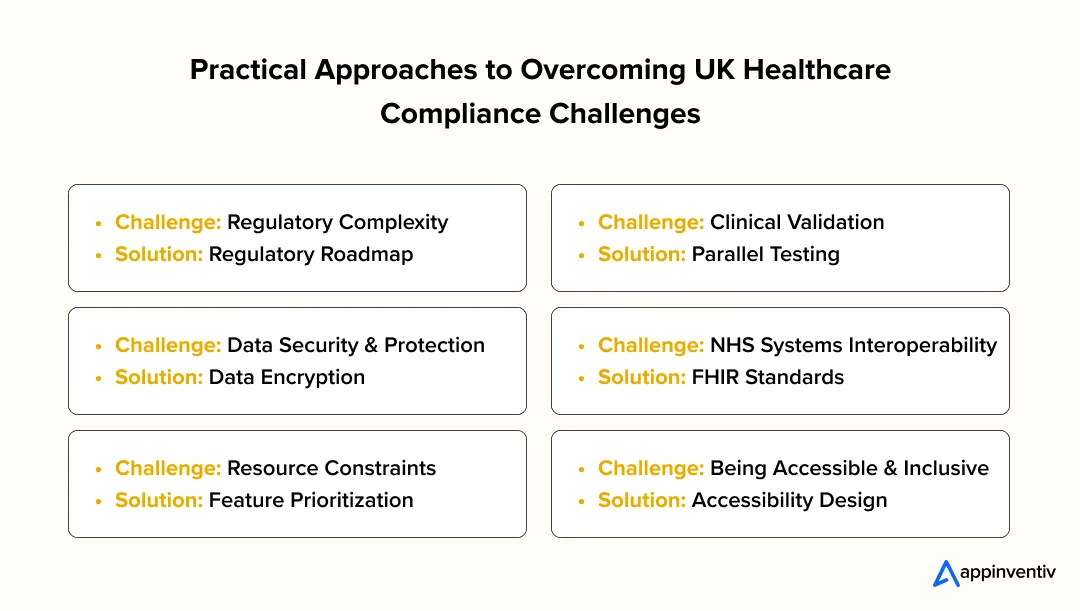
1. Regulatory Complexity
The UK healthcare regulatory environment comprises a range of frameworks, including the UK MDR 2002, MHRA guidance, and NHS Digital standards. So, the requirements overlap may be confusing and slow development.
Solution: Design an elaborate roadmap of regulations at the very beginning. Assign every requirement to app capabilities and software classification, and establish compliance checkpoints at every development stage. Engaging experts can help navigate regulatory challenges in digital health in the UK efficiently.
2. Clinical Validation and Evidence Generation
It is usually difficult to prove the clinical safety and efficacy of new digital solutions, especially with new interventions.
Solution: Parallel clinical tests, usability tests, and pilot programs. Using practical evidence improves the adoption case and regulatory approvals for your solution.
3. Data Security and Data Protection
The security of sensitive patient information is threatened by breaches or misuse, and compliance with data protection laws is imperative.
Solution: The use of strong health data security UK measures, such as encryption, secure authentication, and anonymization. Transparent consent administration and periodic audits would guarantee client loyalty and legal compliance.
4. NHS Systems Interoperability
Applications that are not compatible with the existing NHS infrastructure usually have barriers to adoption.
Solution: Use open standards such as FHIR to ensure interoperability among NHS health apps. Early coordination with NHS Digital stakeholders helps determine technical needs and integration directions.
5. Resource Constraints
Smaller development teams may struggle to meet the financial and technical demands of regulatory compliance, clinical validation, and ongoing monitoring.
Solution: Push compliance-critical features to the forefront, develop them in stages, and find collaborators with NHS organizations or higher education. The strategy balances organizational resource constraints and obligations to high-quality compliance.
6. Being Accessible and Inclusive
Digital solutions should be able to reach large populations of patients with disabilities or low digital literacy.
Solution: Build apps in an accessibility-friendly way to enable inclusive digital healthcare in the UK. Carry out usability testing on different patient groups to guarantee fair access.
Work with our experts to align innovation with compliance while maintaining affordability and operational focus
How to Handle False or Misleading Claims in Healthtech App Marketing
When an organization enters the digital health market by hiring a tech partner, responsible communication is an essential part of UK healthtech app compliance. Marketing groups should realize that regulatory controls are not limited to data treatment but also the manner in which the advantages and functions of an app are to be marketed to the masses. False or exaggerated claims may lead to reputational and legal sanctions in the Advertising Standards Authority (ASA) and in Article 7 of MDR/IVDR.
Here are some of the steps you need to follow for responsible healthtech marketing:
Substantiate Every Claim: Ensure that clinical benefits, diagnostic accuracy, or outcome improvements are supported by verifiable studies or approved data sources. Do not make assumptions or anecdotal findings when explaining performance.
Avoid Implicit Endorsements: Should not mention NHS partnerships, clinical trials, or even medical validations, unless there are formal approvals. The use of phrases such as ‘NHS certified’ or ‘clinically tested’ without any supporting documentation is a violation of advertising rules.
Communication App Limitations: Define the purpose of use, data limitations, and non-clinical features when applicable. This is a way of controlling the expectations of the users, and this shows ethical transparency.
Congruent Messaging and Regulatory Structures: Indicate ASA and MDR/IVDR marketing regulations by comparing your content to these requirements as a part of your UK healthtech app compliance strategy. This guarantees the brand’s credibility and safeguards against regulatory action.
Trust Appinventiv to Align Your HealthTech App with Regulatory Standards
The future of healthtech apps in the UK is about to change in a big way. The main drivers here are the need for secure, efficient, and truly patient-focused digital tools. As the rules get tighter and patients expect more, it’s not enough to just follow the regulations; you have to deliver a smooth user experience. That’s essential. Appinventiv is right at the forefront of this shift, helping healthtech pioneers confidently navigate the UK’s tricky regulatory system.
We stick to all of the UK’s tough compliance standards. This makes sure that data for both the NHS and private practice is managed securely and responsibly. We cover all the necessary requirements, including things like DCB0129, DTAC, DSPT, Cyber Essentials, and the Data Protection Act. This protects patient data while enabling us to build scalable, reliable app solutions.
Appinventiv has already been a partner on some major healthcare projects, like Soniphi, Health-e-People, and DiabeticU. For example, in the case of DiabeticU, our team created an all-inclusive diabetes management application that is fully compatible with wearable devices. It offers its users customized meal plans, workouts, medicine, and 24-hour blood sugar tracking.

DiabeticU changed the way users with diabetes can manage the disease, enhancing health and quality of life by leveraging real-time data and actionable health insights to help them better control the disease.
We deliver healthcare app development solutions that strike a great balance between innovation and compliance. Our hard work has even been recognized with awards like the Leader in AI Product Engineering & Digital Transformation from The Economic Times. That shows we know how to mix the newest technology with regulatory excellence.
Looking ahead, we are preparing for emerging frameworks such as ISO 42001 for AI systems, Cyber Essentials Plus, and the NHS DSPT’s move towards the Cyber Assessment Framework (CAF), future standards that will further strengthen data protection and governance across the healthcare ecosystem.
We encourage you to see the impact yourself. Watch our client testimonial video to find out how Appinventiv helped healthcare groups achieve their digital goals safely and effectively. Partner with us and leverage our reputable app development services in the UK to ensure that your healthtech app not only survives but thrives in the UK’s changing healthcare world.
Connect with our experts today to make your healthtech app compliance-ready.
FAQs
Q. What are NHS requirements for health apps in the UK?
A. NHS requirements are aimed at guaranteeing the app is safe, interoperable, and user-friendly. Key expectations include:
- Integration with existing NHS systems by NHS login integration.
- Adherence to the standards of accessibility to serve a wide range of patients.
- Corresponding to the NHS Digital models related to clinical safety and technical assurance, including DTAC.
- Hi-tech patient data management practices that safeguard privacy and regulatory requirements.
- Demonstration of usefulness and functionality by demonstration and practical use.
Q. How do I get MHRA approval for my medical app?
A. To be approved by MHRA, the first step is to find out whether your app qualifies as a medical device under the UK MDR 2002. The developers will need to conduct clinical and risk assessments to demonstrate safety and effectiveness.
It is necessary to have a comprehensive technical documentation package for health apps in the UK, including design reports, test reports, and proof of conformity. For more risky apps, they might be reviewed by an approved body. Continuous post-market monitoring ensures the app is maintained at the required regulatory level.
Q. Which regulations apply to developing healthtech apps in the UK?
A. Healthtech apps must comply with multiple regulations to achieve UK healthtech app compliance. They are the UK Medical Device Regulations (UK MDR 2002), MHRA software-as-a-medical-device guidance, NHS Digital standards and DTAC, and GDPR.
Other applicable ISO standards include ISO 13485 on quality management and ISO 27001 on information security. By following these frameworks, apps will be secure, efficient, and legally implementable in the NHS.
Q. What’s the process for UKCA marking for health apps?
The UKCA marking process has a number of important steps:
- Identify the risk of the app as per UK MDR 2002 (Class I, IIa/IIb, or III).
- Write a technical file that contains safety, performance, and clinical evidence.
- Perform a conformity check, possibly with the involvement of an approved body for more risky applications.
- Provide a document to MHRA and get authorized.
- Mark the UKCA mark prior to sale in Great Britain to indicate compliance with UK healthtech apps.
Q. How does GDPR affect medical app development in the UK?
A. GDPR proposes stringent regulations on the management of patient data. The developers should make sure that they provide secure storage, encryption, and clear consent management procedures. Audits and privacy impact assessments should be conducted regularly to ensure compliance. These measures will address health data security UK and provide a guarantee of patient information security during the lifecycle of the app.
Q. What makes a healthcare app legally compliant in the UK?
A. Legal issues demand that the standards of MHRA regulations, NHS Digital standards, and GDPR are satisfied. Apps should be clinically safe, have appropriate records, and be interoperable with NHS systems where necessary.
Meeting accessibility standards for NHS apps will ensure that patients, including those with disabilities or low levels of digital literacy, can use the app safely and effectively. The documentation and evidence of such measures are of paramount importance to get the legal approval.
Q. How are medical devices classified for apps in the UK?
A. Medical devices, software included, can be categorized according to risk: Class I (low risk), Class IIa/ IIb (medium risk), and Class III (high risk). Classification helps to define the degree of regulatory attention, testing, and documentation that is required to obtain approval. Proper classification can also assist in guaranteeing interoperability of NHS health apps through the synchronization of technical and clinical requirements and NHS requirements.


- In just 2 mins you will get a response
- Your idea is 100% protected by our Non Disclosure Agreement.
How to Build a Custom Pediatric EMR and EHR System?
Key takeaways: Clinical Precision: Custom systems accommodate pediatric-specific data points like percentile curves and weight-based longitudinal dosing. Interoperability: Seamless data exchange via HL7 FHIR ensures your practice stays connected to pharmacies, labs, and state registries. Regulatory Resilience: Built-in compliance with HIPAA, HITECH, and MACRA/MIPS reduces legal friction. Enhanced Engagement: Parent portals reduce administrative overhead by…

Change Management in Healthcare: Principles, Processes, and Models
Key Takeaways Change in healthcare fails quietly when ownership, workflow alignment, and follow-through are missing. Successful change management in healthcare focuses on adoption, not just system implementation. Clinical workflows and workforce capacity determine whether transformation sticks or stalls. Governance, clear accountability, and post-go-live support matter more than the model used. Sustainable healthcare transformation depends on…
A Practical Guide to Building Your Mental Health Chatbot - Use Cases, Cost, & ROI
Key takeaways: Mental health chatbots work when they know their limits. They’re most useful as a gentle first step, not as a stand-in for real care. Good chatbot design is more about judgment than AI. Clear boundaries, calm responses, and safety matter more than smart language models. Enterprises invest in chatbots to make support easier…